Metallurgy of Some Important Metals
Metallurgy of Some Important Metals: Overview
This topic covers concepts, such as, Extraction of Aluminum, Extraction of Iron, Electrolytic Reduction Method & Flux etc.
Important Questions on Metallurgy of Some Important Metals
What are the refractory materials and suggest few examples?
Write the equation for getting copper from low grade ore.
Write the process of extraction of copper from low grade ores and scraps.
The slag formed from the blast furnace during the extraction of iron is used to manufacture high temperature resistant building products.
The slag formed from the blast furnace during the extraction of iron is used to manufacture Portland cement.
Mention few uses of slag formed from the blast furnace during the extraction of iron.
Wrought iron is formed in a _____ furnace.
Which of the following is the purest form of iron?
How is wrought iron produced.
What are the characteristics of wrought iron?
Write the composition of wrought iron.
What is wrought iron?
During the extraction of haematite, limestone is added which acts as _____.(flux/slag)
During the extraction of haematite, limestone is added which acts as
In Copper extraction process, the ore is heated in a reverberatory furnace after mixing with silica. In the furnace, iron oxide ‘slags of’ as _____ silicate and copper is produced in the form of copper matte. (Give common name of the substance.)
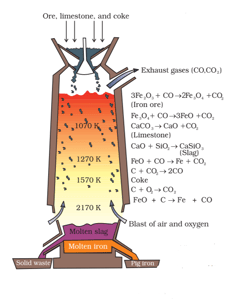
The above process diagram of a machinery that is used in the extraction of iron. The diagram is of a _____.
Blister copper is :
The above reactions convert Copper Sulphide/Oxide into blister copper. The total number of copper in the product side of the reactions are:
Bassemerization is used in the extraction of:
The role of graphite in the electrometallurgy of aluminium is: